
In recent years, the development of induced pluripotent stem cell (iPSC) technology has been profoundly changing the way we explore the mysteries of the brain. Researchers no longer rely solely on animal models or limited patient samples. Instead, they are able to construct human nerve cells and even miniature "brain-like" structures in petri dishes using IPscs, opening up unprecedented doors for understanding neurological diseases and developing new therapies. From the initial simple two-dimensional cell sheets to today's three-dimensional neural spheres and brain-like organs capable of simulating the complex structure and function of the brain, the development of iPSC neural models has been advancing rapidly.
Recently, several studies have once again refreshed our understanding of the potential of IPscs, pushing the construction and application of neural models to a new height. This article will focus on three representative achievements. The first One is the study published in PLoS One by Rhea Sullivan and her team from the School of Medicine at Pennsylvania State University in the United States. They constructed a midbrain neural stem cell model using IPscs to explore the profound impact of prenatal opioid exposure on brain development. Immediately after that, the team of Sarah De Beuckeleer from the University of Antwerp in Belgium presented their innovative achievements at eLife. They developed an image analysis method based on artificial intelligence, which can accurately identify different cell types in complex neural cultures, greatly enhancing the accuracy and efficiency of the research. Finally, the team of Tongguang Wang from the National Institute of Neurological Disorders and Stroke in the United States published their new protocol for constructing human brain organoids containing microglia at JoVE, providing a more realistic platform for studying immune responses and neuroinflammation in the brain. The above research reflects the exciting development prospects of iPSC technology in the field of neuroscience, indicating that we are one step closer to the goal of conquering stubborn diseases of the nervous system.
Research highlights
1. The iPSC model was the first to precisely simulate the specific effects of prenatal opioid exposure and withdrawal on the fate of human cranial nerve progenitor cells, revealing the potential mechanism of drug intervention in development.
2. AI empowers cell recognition. The CNN tool it has developed can accurately and unbiased analyze the components of complex IPSC-derived neural cultures, enhancing the efficiency of model quality control and analysis.
3. Human brain organoids containing endogenous developmental microglia have been successfully constructed, providing a more realistic platform for the study of early neuro-immune interactions and related diseases.




Content Introduction
01 Simulating "Pregnancy Risks" : iPSC reveals the impact of opioid drugs on the developing brain
The potential harm of prenatal opioid exposure to neonatal neurodevelopment has become a public health issue of increasing concern. To explore this issue at the cellular level, Sullivan R from the School of Medicine at Pennsylvania State University The team ingeniously utilized the iPSC technology to construct a human midbrain neural progenitor cell model. They first differentiated the IPscs of healthy people into neural progenitor cells in the midbrain region, which are the "seeds" of key neurons (such as dopamine neurons) in the future brain.
The research team meticulously optimized the differentiation protocol to ensure that these progenitor cells could fully express the key receptors of opioid drugs, thereby more realistically simulating the targets of drug action. Subsequently, they exposed these cells to chronic morphine in a culture dish and simulated the process of drug withdrawal. Through a series of precise cellular and molecular biological tests, they found that compared with the control group and the pure morphine exposure group, neural progenate cells that underwent morphine withdrawal showed significant "overshoot" at cyclic adenosine monophosphate (cAMP) levels - a common biochemical signaling change in opioid withdrawal in mature neurons. It indicates the sensitivity of nerve cells to drug withdrawal during the early developmental stage.
More importantly, the research has revealed the profound impact of drug exposure and withdrawal on cellular fate. Chronic morphine exposure seems to "jam" the development process of neural progenitor cells, causing more cells to remain in the immature progenitor cell state (an increase in the proportion of nestin positive cells), while the proportion of differentiated and mature neurons (a decrease in the proportion of NeuN positive cells) correspondingly decreases. However, when morphine was withdrawn (simulated withdrawal), the situation reversed: the proportion of astrocytes (GFAP-positive cells, important supporting cells in the brain) decreased, while the proportion of neurons increased significantly, even exceeding the normal level. This discovery suggests that prenatal exposure and withdrawal of opioid drugs may disrupt the delicate balance produced by neurons and glial cells, potentially having long-term effects on the early construction of the brain. Although no significant changes in cell proliferation and overall viability were observed in this study, the in vitro model it established provides a valuable tool for in-depth research on how opioids affect cell fate determination in early neurodevelopment and lays the foundation for future exploration of intervention strategies (Figure 1).

Figure 1. Chronic morphine exposure and withdrawal have altered the proportion of final differentiation of lineage-specific midbrain progenitor cells. (a) Experimental procedure for chronic morphine exposure and withdrawal treatment using midbrain nerve progenitor cells obtained by longer nerve induction and modeling methods. (b) The differentiated neural progenitor cells were fixed and intracellular cAMP immunostaining was performed 24 hours after morphine withdrawal (n=3). The scale is 300 μm. (c) The average fluorescence intensity values of cAMP under different conditions were quantified using Biotek Cytation 5. Compared with the vector group (VEH) and the morphine exposure group (MOR), the average fluorescence intensity of cAMP in the withdrawal group (WD) cells was significantly higher (one-way analysis of variance, Tukey multiple comparisons, F = 9.411, p = 0.014). (d) There was no difference in the relative expression level of the proliferation marker Ki67 under different conditions (one-way analysis of variance, F = 0.2614, p = 0.7783). (e) On the 56th day, the differentiated cells were fixed and immunostained with cell identity markers NES (green), GFAP (orange), and NEUN (red). All the images were taken under a 10x microscope. The scale is 300 μm. (f) The cell identity was determined by setting a threshold for the average staining intensity of each DAPI+ cell using Biotek Cytation 5. The proportion is determined by dividing the total number of NES+, GFAP+ or NEUN+ cells by the total number of DAPI+ cells. There was a statistically significant interaction in the effects of different treatment conditions and cell markers on the proportion of differentiated cell fate (two-factor analysis of variance, F(4,18) = 16.05, p < 0.0001). (g) Chronic morphine exposure and withdrawal did not lead to changes in the transcriptional level of the classical opioid receptor (two-factor analysis of variance, F(4,18) = 0.569, p = 0.688). (h) LIVE/DEAD™ staining was performed on live and unfixed cells 24 hours after morphine withdrawal. The scale is 300 μm. (i) There was no difference in the proportion of calcein-AM + cells under different treatment conditions (one-way analysis of variance, F = 0.292, p = 0.756).
02 AI's Discerning Eye for Cells: Intelligently analyzing complex neural cultures
As IPSC-derived neural models become increasingly complex, such as mixed cultures or three-dimensional organoids containing multiple types of nerve cells, how to accurately and efficiently identify and quantify the different cellular components within them has become a huge challenge. Traditional manual counting or analytical methods based on specific markers are not only time-consuming and labor-intensive, but sometimes also difficult to deal with complex situations such as high cell density and large morphological heterogeneity. Regarding this difficult problem, De Beuckeleer S from the University of Antwerp The team has developed an innovative solution based on artificial intelligence (AI).
Their core technology is an image analysis method that combines "cell painting" (a multi-channel fluorescence imaging technique) and convolutional neural networks (CNNs). Researchers first used a variety of fluorescent dyes to stain the cultured nerve cells, capturing the morphology, texture and expression information of key proteins of the cells. Then, they trained the CNN model to learn and identify the unique "fingerprints" of different cell types. Impressiingly, this AI method achieved an accuracy rate of over 96% in differentiating neuroblastoma and astrocytoma cell lines, significantly outperforming the traditional random forest classification method based on morphological and texture features. The team also proposed a clever "nucleocentric" analysis strategy. In cultures with very dense cells, it is extremely difficult to completely segment the contours of individual cells. The "nuclear center" method, on the other hand, focuses the analysis on the cell nucleus and its adjacent surrounding areas. It utilizes the relatively easily identifiable and information-rich regional features of this part to determine the cell type, thereby maintaining high accuracy even under high-density culture conditions.
More practically significant is that this method has been successfully applied to real neural cultures derived from IPscs. It can not only accurately assess the mature state of the culture (with an accuracy rate as high as 96% in distinguishing neurons from progenitor cells), but also precisely distinguish different types of cells in complex mixed cultures containing neurons, astrocytes and microglia, and even identify different activation states of microglia. The breakthrough of this technology provides a powerful automated tool for the quality control, component analysis and high-throughput drug screening of iPSC neural models, which is expected to greatly enhance the repeatability and cell type specificity of research and accelerate the research process in the field of neuroscience (Figure 2).
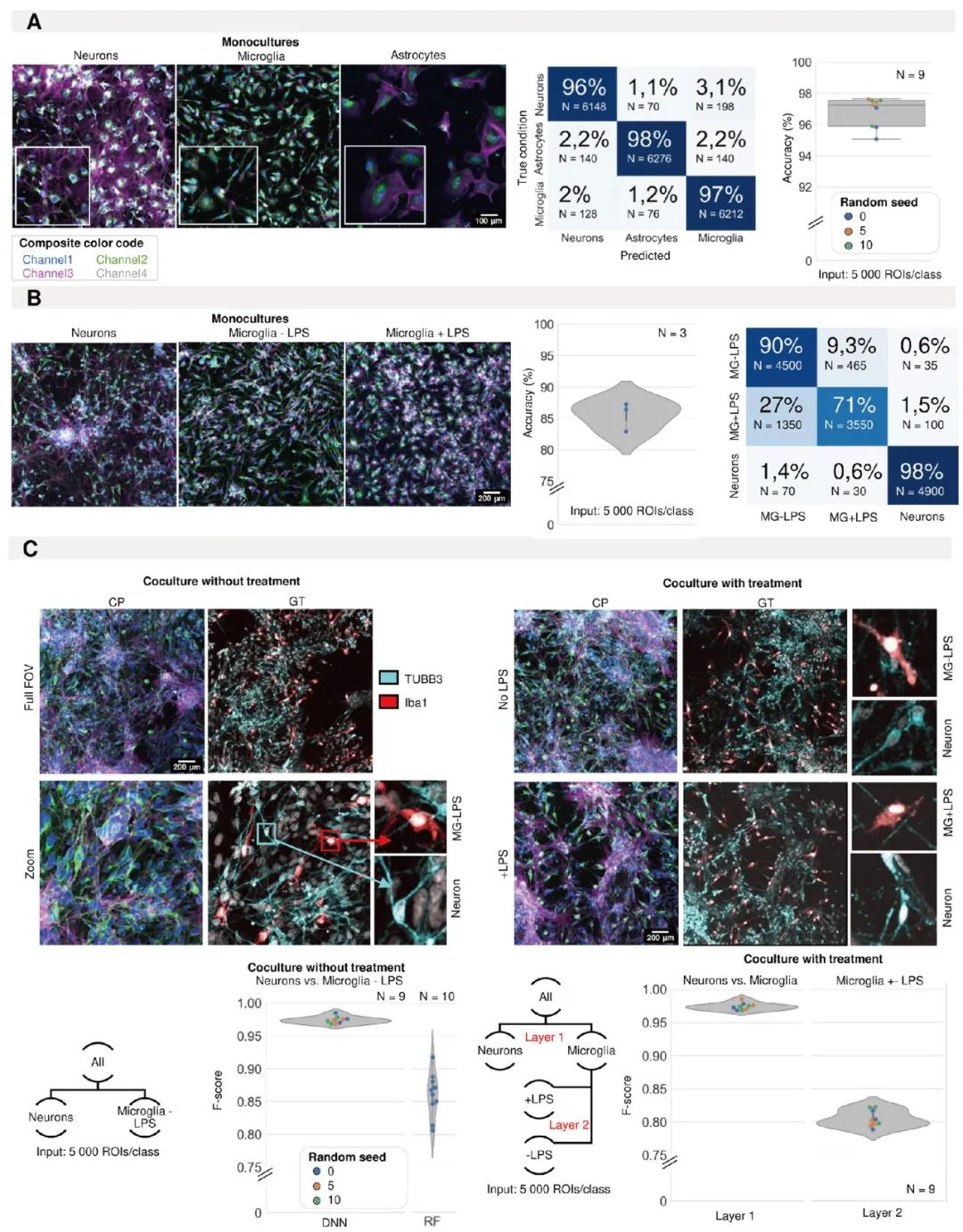
Figure 2. Identification of induced pluripotent stem cell (iPSC) cell types using morphological analysis. (A) Representative images of IPSC-derived neurons, astrocytes and microglia with morphological staining in a single culture. The prediction accuracy and confusion matrix (average of all models) of convolutional neural network (CNN) for classifying single cultures of IPSC-derived astrocytes, microglia and neurons. Each point in the box plot represents the F1 score of a classifier (model initialization, N=9). The classifier was trained three times using three different random seeds. (B) Representative images of IPSC-derived single cultures of neurons and microglia treated with LPS or control (color code definitions are shown in Figure 1A). The prediction accuracy and confusion matrix (the average of all models) are given. Each point in the violin graph represents the F1 score of a classifier (model initialization, N=3). (C) Representative images of mixed culture of IPSC-derived microglia and neurons. The real situation was identified by immunofluorescence staining (IF). Each point in the violin graph represents the F1 score of a classifier (model initialization). The classifier is trained using three different random seeds. The results of CNN were compared with those of shallow learning (random forest, RF). The same analysis was conducted on the mixed cultures of neurons and microglia treated with LPS or control treatment. The stratified method was adopted. Firstly, neurons were separated from microglia, and then the treated and untreated microglia were classified. Each point in the violin graph represents the F1 score of a classifier (model initialization, N=9). The classifier was trained three times using three different random seeds.
03 Constructing a "mini immune brain" : iPSC organoids integrate microglia
Brain organoids, these "miniature brains" formed by three-dimensional in vitro culture of IPscs, provide an unprecedented platform for simulating human brain development and diseases. However, most of the early brain organoid models were missing a key cellular component - microglia. Microglia are resident immune cells in the brain and play a crucial role in neuroinflammation, neuroinfection, as well as brain development and homeostasis maintenance. How to integrate functional microglia in organoids has always been the direction of efforts in this field.
The team of Wang T. from the National Institute of Neurological Disorders and Stroke in the United States has made significant progress in this field. They have developed a reliable and repeatable protocol for generating human brain organoids with endogenous developmental microglia. The core strategy is to integrate IPSC-derived hematopoietic progenitor cells (HPCs, the precursor cells of microglia) into ipscs at the stage when they differentiate into embryoids (the early prototypes of organoids). Subsequently, these co-cultured embryoids gradually developed into three-dimensional brain organoids containing neurons and microglia under specific neural induction, proliferation and maturation conditions.
Through advanced techniques such as immunostaining (for detecting microglial cell-specific markers like IBA1 and TREM2) and single-cell RNA sequencing, researchers confirmed that microglia developed from the co-introduced HPCs do exist within these organoids. More importantly, these integrated microglia are not "all show and no substance"; they have normal physiological functions. When these organoids were stimulated with lipopolysaccharide (LPS, a substance that can simulate bacterial infections and induce inflammatory responses), the microglia within them exhibited typical inflammatory responses, demonstrating their immune activity. Interestingly, even without adding all the specific cytokines known to promote microglial differentiation to the culture medium, HPCs were still able to successfully differentiate into microglial cells in the neural environment of organoids. This indicates that the developing neural microenvironment itself can generate sufficient signals to guide the specialization and maturation of microglia, which makes the model closer to the real brain development process in vivo. This work not only provides a valuable tool for studying the early interactions between the innate immune system and the developing nervous system, but also opens up new avenues for simulating complex diseases involving neuroinflammation or neuroinfections (such as Alzheimer's disease, Parkinson's disease, Zika virus infection, etc.) (Figure 3-4).
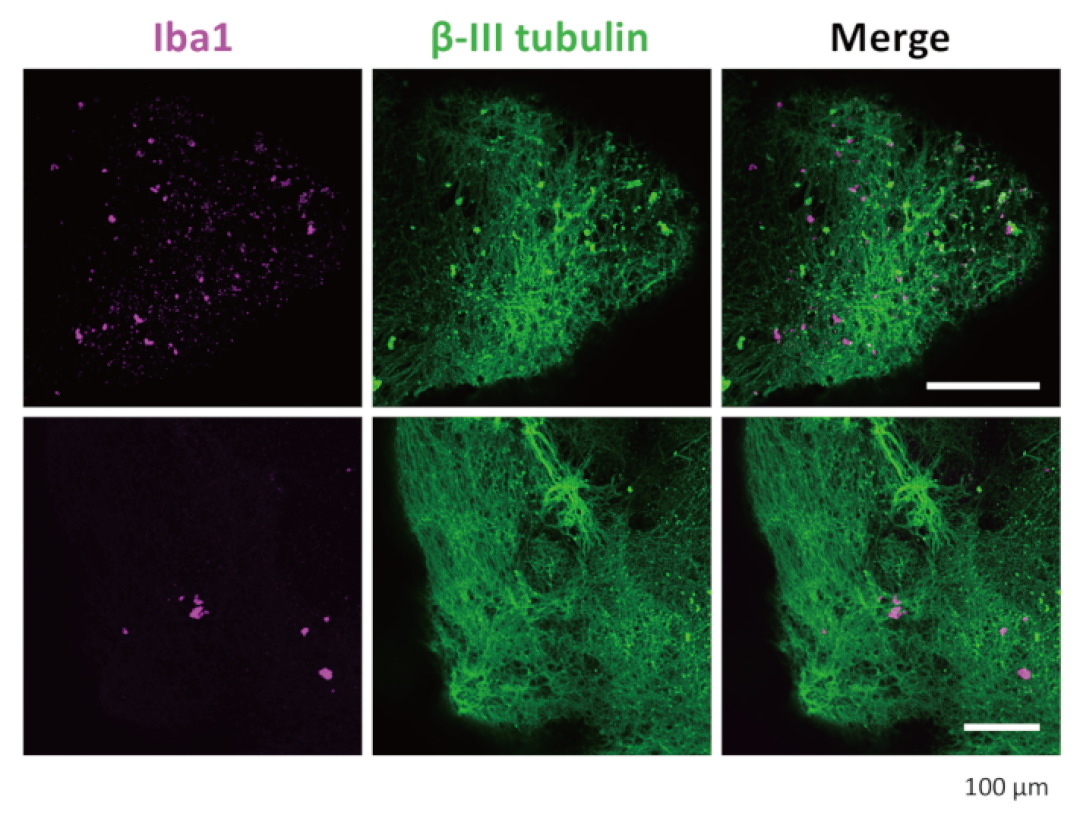
Figure 3.3 Immunostaining of neurons and microglia in D-organoids. After transparency treatment and immunostaining, the organoids showed that microglia were positively stained with IBA1 and neurons were positively stained with β III-tubulin. The representative images displayed were captured under a confocal microscope using a 20x objective lens.
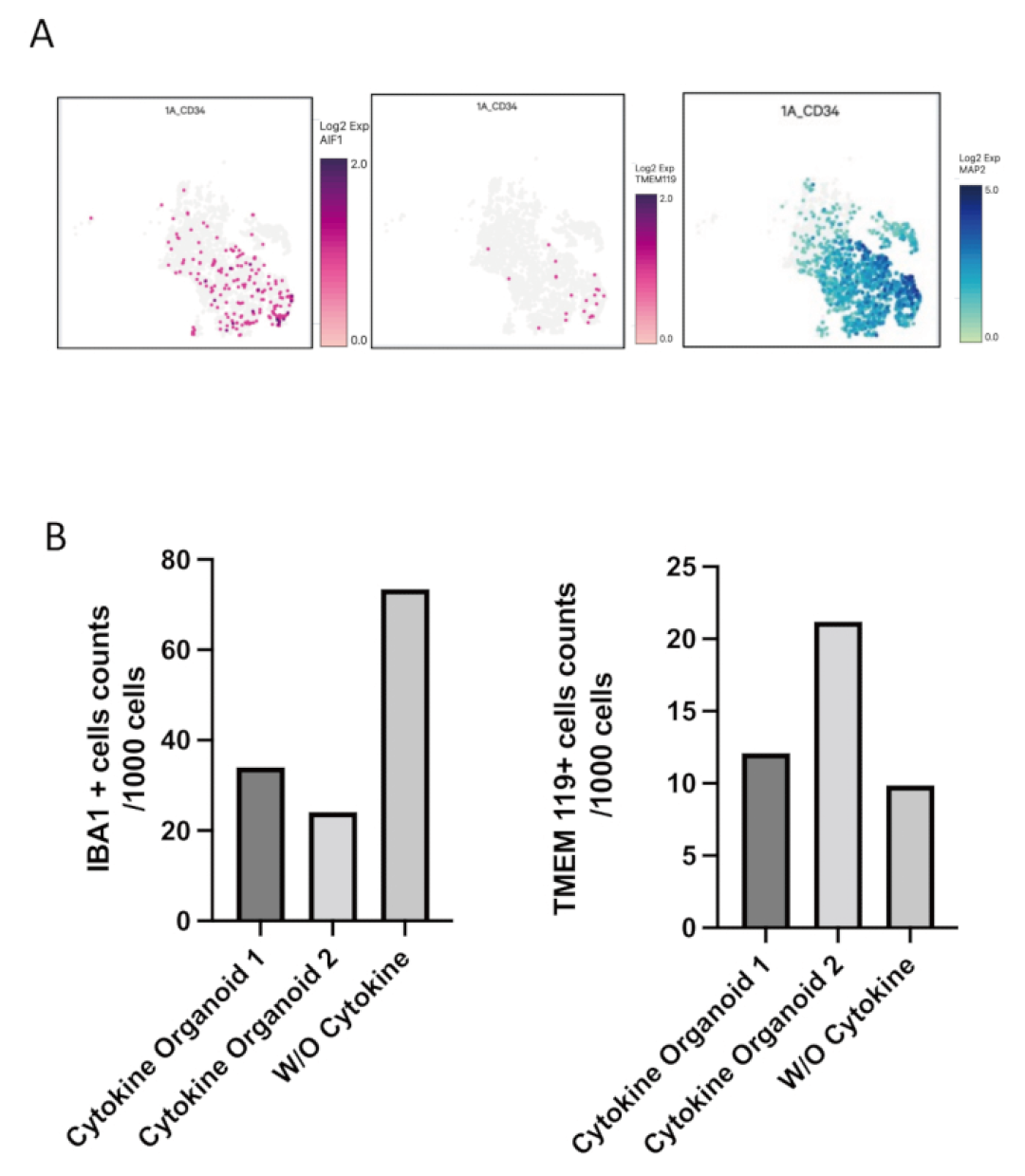
Figure 4. Single-cell RNA sequencing results of 3D organoids containing microglia. (A) The chart shows the distribution of IBA1(AIF1), TMEM119 and MAP2 in organoids without the addition of additional microglial differentiation cytokines. (B) Microglia positive for IBA1 and TMEM119 were counted in two organoids cultured with additional microglial differentiated cytokines and one organoid not cultured with them. The number of IBA1 and TMEM119 positive cells in representative organoids has changed.
Future Outlook
In the future, IPSC-induced neural models will still be full of infinite possibilities and will continue to shine in multiple fields of neuroscience. Firstly, in the direction of model construction, scientists will focus on enhancing the "maturity" of differentiated cells to make them closer to the physiological state of adults, thereby more accurately simulating late-onset neurodegenerative diseases. Meanwhile, constructing three-dimensional brain-like organs that contain more types of cells (such as vascular cells and more types of immune cells), have more complex structures and more complete functions, will be an important research direction in the future. In addition, establishing a standardized production and identification process for iPSC nerve cells is crucial for ensuring the reliability and repeatability of research results, as well as meeting the demands for cell quantity and quality in large-scale drug screening and future clinical treatments. Technological integration will be the main theme driving future development. AI and machine learning will play an increasingly important role in analyzing the massive and complex data generated by iPSC models (such as high-content imaging and multi-omics data) and optimizing cell differentiation schemes.
The development of iPSC technology is also accompanied by challenges such as production costs, potential immune rejection risks (even in autologous cells, this may occur under specific circumstances), and long-term safety and efficacy. However, iPSC technology and its derived neural models undoubtedly bring us an unprecedentedly bright prospect for understanding and overcoming neurological diseases. These constantly evolving "palm brains" are helping scientists uncover the mysteries of the brain with unprecedented depth and breadth.